Abstract
Introduction
Statins are a groups of drugs used in the treatment of hyperlidemia and in primary and secondary prevention of cardiovascular disease. Recently, there has been increasing interest in statins for their ability to improve survival and reduce risk of cancer due to their pleiotropic effects. Observational studies have reported survival benefit among patients with several cancers including pancreatic cancer, ovarian cancer and colorectal cancer. It has also been shown to reduce incidence risk in hematologic malignancies including lymphoid neoplasms. Recently, Sanfillipo et al (J Clin Oncol 2016 34:4008-4014) reported that statin use among patients with Multiple Myeloma (MM) was associated with decreased mortality in a Veteran Affairs (VA) database. We attempted to replicate these findings among older patients with MM using data from SEER-Medicare database.
Methods
All cases of MM diagnosed 2008-2013 in the SEER-Medicare claims were extracted along with their associated Medicare claims data from 2007-2014. Incomplete cases including: those diagnosed prior to age 65, death certificate or autopsy cases, and those not enrolled in Medicare Part A (inpatient), B (outpatient), and D (prescription) were excluded. Cases of suspected smoldering MM, those without MM defining "CRAB" symptoms and not treated within 6 months following diagnosis, were excluded using a pre-established algorithm (Fakhri, et al,Blood 2016 128:2348). Statin users, defined as having 2 or more prescriptions for statins within 1 year before and after diagnosis of MM, were identified by Part D claims. ICD-9 codes were used to identify patients with chronic kidney disease (CKD). Charlson comorbidity index (CCI) was calculated using a previously established algorithm. Receipt of novel MM agents as first-line therapy and stem cell transplant and bisphosphonate utilization within 1 year following diagnosis were defined as the presence or absence of the corresponding ICD-9 and HCPCS codes. Baseline characteristics were compared between statin users and non-users by Chi-square test and Student's T-test as appropriate. Multivariate analysis was performed by Cox proportional hazard modeling method. Statin exposure was also assessed as a time-dependent covariate to account for immortal time bias. All statistical analysis was performed using SAS software 9.4 (Cary, NC, USA)
Results
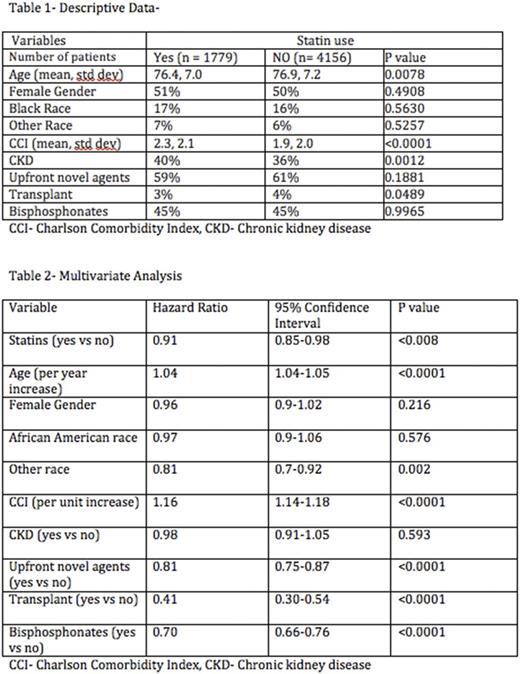
5935 patients were included in the study of which 1779 were statin users. Descriptive data is shown in table 1. Statin users tended to be slightly older, had a higher rate of CKD and higher mean CCI, but were slightly more likely to undergo ASCT (Autologous stem cell transplant). In the survival analysis, statin use (time-dependent) was associated with decreased mortality with an adjusted hazard ratio of (aHR- 0.91, 0.85-0.98, p=0.008). Use of novel agents, bisphosphonates, and stem cell transplant were all associated with decreased mortality; increasing age and CCI were associated with increased mortality. The full multivariate results are shown in table 2.
Conclusions
Statins as a class of drugs exert their effect on mevalonate pathway by inhibiting HMG-CoA reductase for their cholesterol lowering effect and this disruption of mevalonate pathway has also been shown to induce apoptosis in MM cells. In vitro studies of MM cells have also shown to induce cell death and decrease proliferation when exposed to statins. This susceptibility of MM cells to statins in laboratory experiments has been further supported by findings in retrospective studies with Epstein et al (Int. J. Cancer 2017 141: 480-487) reporting reduced incidence of MM with statin use and Sanfillipo et al showing decreased mortality with statin use among patients with MM.
In the present study we found that statin use decreased mortality by 9%. Our results are similar to the findings reported by Sanfillipo et al and further support a potential benefit of statin use among patients with MM. These results should be evaluated further in prospective clinical trials.
Vij: Celgene: Honoraria; Konypharma: Honoraria; Takeda: Honoraria, Research Funding; Janssen: Honoraria; Bristol-Meyers-Squibb: Honoraria; Jazz: Honoraria; Abbvie: Honoraria; Amgen: Honoraria, Research Funding. Wildes: Carevive Systems Inc: Consultancy; Janssen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal